 In the ever-evolving landscape of antimicrobial resistance, health care providers and infectious diseases specialists are frequently confronted with formidable challenges in treating infections caused by extended-spectrum beta-lactamase–producing and carbapenem-resistant Enterobacteriaceae. Although the national incidence of CRE infections in the United States remains relatively low, this threat continues to loom large as a significant concern within health care systems, primarily due to the limited availability of effective antimicrobial treatments.
In the ever-evolving landscape of antimicrobial resistance, health care providers and infectious diseases specialists are frequently confronted with formidable challenges in treating infections caused by extended-spectrum beta-lactamase–producing and carbapenem-resistant Enterobacteriaceae. Although the national incidence of CRE infections in the United States remains relatively low, this threat continues to loom large as a significant concern within health care systems, primarily due to the limited availability of effective antimicrobial treatments.
Research examining outcomes in patients infected with CRE is also notably scant, often characterized by small-scale studies that yield conflicting results. (1) In management of these complex infections, certain time-honored antibiotics, such as fluoroquinolones (ciprofloxacin or levofloxacin) and trimethoprim-sulfamethoxazole, may yet hold their ground as viable options under specific circumstances.
While the new generation cephalosporins and contemporary beta-lactam combinations are usually preferred for empirical treatment within the contexts of ESBL and CRE, all antibiotics should be evaluated equitably and streamlined to create more straightforward regimens, guided by the tenets of antibiotic stewardship. Current guidelines propose that oral step-down therapy can be used when applicable for infections (pyelonephritis, intra-abdominal infections and bacteremia) with ESBL. However, there is very limited data to support the application of the same strategy in managing complicated CRE infections (beyond pyelonephritis) even when good susceptibilities to fluoroquinolones or TMP-SMX are identified. (1)
In the battle against gram-negative bacteremia, a humble pill has been described as standing toe-to-toe with its intravenous counterpart. (2) Theoretically, this approach seems plausible, yet the critical question remains: Who would dare to take such a risk with CRE bacteremia?
In alignment with the “Oral is the new IV” dogma, this conundrum necessitates further research. This shift would not only enhance patient comfort and convenience but also would mitigate the risk of complications associated with IV lines. Moreover, the judicious use of oral antibiotics conserves hospital resources and reduces health care costs. By optimizing the use of oral antibiotics when clinically appropriate, we can maintain the efficacy of existing treatments, decrease the selection pressure for resistant strains and uphold the principles of responsible antibiotic use. (3)
 The urgent need for effective oral antibiotics to treat multidrug-resistant gram-negative Enterobacteriaceae remains unmet. Even after being studied in ADAPT-PO trial, the oral carbapenem tebipenem has not yet satisfied Food and Drug Administration requirements for approval as an alternative treatment option in ESBL infections, leaving a critical gap in our oral antibiotic arsenal. (4) Hope remains on the horizon with FDA’s approval of oral pivmecillinam, a medicine used in Europe for decades and now poised to combat multidrug-resistant urinary tract infections afflicting millions of Americans annually. (5) However, this development highlights a bittersweet reality: We still lack effective new oral options for complicated infections such as bacteremia, let alone CRE.
The urgent need for effective oral antibiotics to treat multidrug-resistant gram-negative Enterobacteriaceae remains unmet. Even after being studied in ADAPT-PO trial, the oral carbapenem tebipenem has not yet satisfied Food and Drug Administration requirements for approval as an alternative treatment option in ESBL infections, leaving a critical gap in our oral antibiotic arsenal. (4) Hope remains on the horizon with FDA’s approval of oral pivmecillinam, a medicine used in Europe for decades and now poised to combat multidrug-resistant urinary tract infections afflicting millions of Americans annually. (5) However, this development highlights a bittersweet reality: We still lack effective new oral options for complicated infections such as bacteremia, let alone CRE.
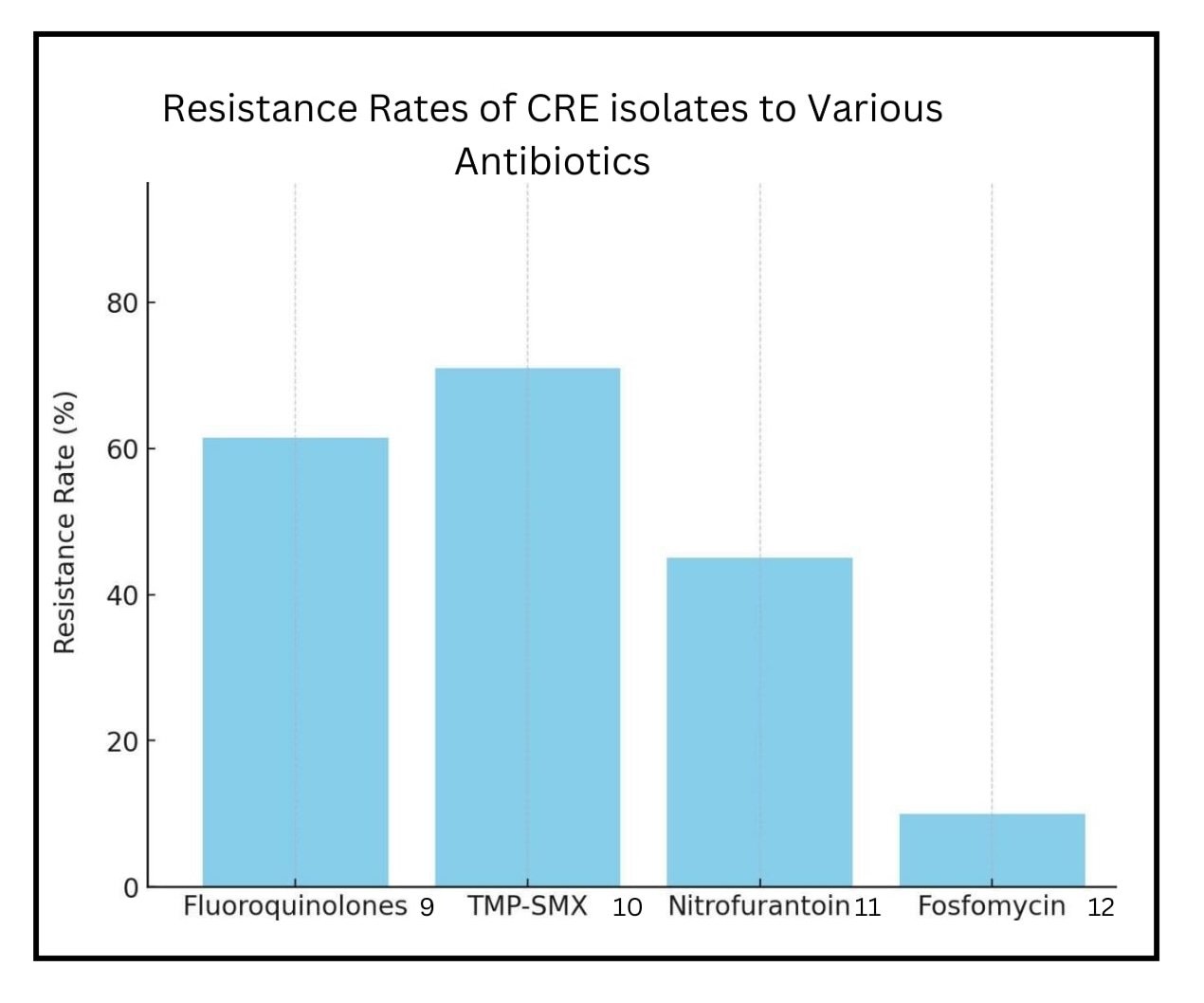
Sparse literature suggests that fluoroquinolones or TMP-SMX can be used for CRE pathogens in complicated infections such as bacteremia beyond urinary tract infections and pyelonephritis, contingent upon susceptibility testing confirming their efficacy. (6-8) (Figure) However, the evidence remains anecdotal, requiring more robust clinical trials to instill confidence among infectious diseases specialists, which could enable them to embrace these established drugs without hesitation in CRE infections.
References
- Livorsi DJ, Chorazy ML, Schweizer ML, et al. A systematic review of the epidemiology of carbapenem-resistant Enterobacteriaceae in the United States. Antimicrob Resist Infect Control. 2018;7:55. Published 2018 Apr 24. doi:10.1186/s13756-018-0346-9
- Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America Antimicrobial-Resistant Treatment Guidance: Gram-Negative Bacterial Infections. Infectious Diseases Society of America 2023; Version 3.0.
- Tingsgård S, Bastrup Israelsen S, Jørgensen HL, Østergaard C, Benfield T. Early Switch From Intravenous to Oral Antibiotics for Patients With Uncomplicated Gram-Negative Bacteremia. JAMA Netw Open. 2024;7(1):e2352314. Published 2024 Jan 2. doi:10.1001/jamanetworkopen.2023.52314
- Bolton WJ, Wilson R, Gilchrist M, Georgiou P, Holmes A, Rawson TM. Personalising intravenous to oral antibiotic switch decision making through fair interpretable machine learning. Nat Commun. 2024;15(1):506. Published 2024 Jan 13. doi:10.1038/s41467-024-44740-2
- Eckburg PB, Muir L, Critchley IA, et al. Oral Tebipenem Pivoxil Hydrobromide in Complicated Urinary Tract Infection. N Engl J Med. 2022;386(14):1327-1338. doi:10.1056/NEJMoa2105462
- Lodise TP, Kaye KS, Santerre Henriksen A, Kahlmeter G. Review of the In Vitro Microbiological Activity of Mecillinam Against Common Uropathogens in Uncomplicated Urinary Tract Infection: Focus on Resistant Pathogens. Open Forum Infect Dis. 2024;11(6):ofae296. Published 2024 May 24. doi:10.1093/ofid/ofae296
- Luterbach CL, Boshe A, Henderson HI, et al. The Role of Trimethoprim/Sulfamethoxazole in the Treatment of Infections Caused by Carbapenem-Resistant Enterobacteriaceae. Open Forum Infect Dis. 2018;6(1):ofy351. Published 2018 Dec 14. doi:10.1093/ofid/ofy351
- Ávila-Núñez M, Lima O, Sousa A, et al. Carbapenem alternatives for treatment of bloodstream infections due to AmpC producing enterobacterales. Ann Clin Microbiol Antimicrob. 2023;22(1):75. Published 2023 Aug 17. doi:10.1186/s12941-023-00624-9
- Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-beta-lactamases: the quiet before the storm?. Clin Microbiol Rev. 2005;18(2):306-325. doi:10.1128/CMR.18.2.306-325.2005
- Luterbach CL, Boshe A, Henderson HI, et al. The Role of Trimethoprim/Sulfamethoxazole in the Treatment of Infections Caused by Carbapenem-Resistant Enterobacteriaceae. Open Forum Infect Dis. 2018;6(1):ofy351. Published 2018 Dec 14. doi:10.1093/ofid/ofy351
- Amladi AU, Abirami B, Devi SM, et al. Susceptibility profile, resistance mechanisms & efficacy ratios of fosfomycin, nitrofurantoin & colistin for carbapenem-resistant Enterobacteriaceaecausing urinary tract infections. Indian J Med Res. 2019;149(2):185-191. doi:10.4103/ijmr.IJMR_2086_17
- Michalopoulos A, Virtzili S, Rafailidis P, Chalevelakis G, Damala M, Falagas ME. Intravenous fosfomycin for the treatment of nosocomial infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients: a prospective evaluation. Clin Microbiol Infect. 2010;16(2):184-186. doi:10.1111/j.1469-0691.2009.02921.x
